All published articles of this journal are available on ScienceDirect.
Humidity Effect on the Photocatalytic Activity of Sustainable Cement-Based Composites
Abstract
Introduction:
In this study, a side-by-side comparison was performed to examine the effects of nano-TiO2 (nT) on the photocatalytic activity of Portland cement (PC) mixtures and its effectiveness compared to similar replacement percentages of micro-TiO2 (mT), both of which were 100% anatase.
Methods:
Cement paste specimens were prepared by progressive cement replacement with nT or mT. To simulate the effect of humidity in different seasons, the PC specimens were subjected to different moisture conditions, including normal conditions with 50% Relative Humidity (RH), saturated conditions with 100% RH, and oven-drying conditions with 0% RH. Their photochemical reaction (i.e., gas removal ability) with TiO2 was evaluated and compared with the control specimens. Furthermore, the self-cleaning ability of the mixtures was examined by applying a drop of methylene blue dye to the specimen surface; differences in dye discolouration with time under different illuminations were noticed.
Results and Conclusion:
This paper describes the results of an attempt to understand the effect of humidity on the photocatalytic performance of PC composites that incorporate TiO2. The results show that PC mixtures modified with nano titanium are more effective in hot and dry conditions. In contrast, micro titanium mixtures appear to be favourable for wet conditions. Furthermore, the particle size (micro versus nano), matrix voids and absorption values of the cement-TiO2 composite were identified as the key factors affecting the photocatalytic performance.
1. INTRODUCTION
Photocatalytic building materials have been used since the 1990s [1] and have received great interest, particularly in Europe and Japan [2]. The photocatalyst materials represent a promising solution to remediate air pollution in urban areas, which demonstrates their effectiveness and sustainability [3, 4]. Most studies used nano-titanium oxide (nT) and Nano-Silica (nS), and few studies used nano-Fe2O3 [5]. The use of titanium dioxide (TiO2) in environmental applications is a good choice because TiO2 1) is chemically stable, safe, and has a relatively low- cost; 2) is compatible with other building substrates; 3) has a reasonable performance even under low solar radiation [6]; 4) self-cleaning ability because of the surface chemical reactions in the presence of sunlight; 5) can form a hydrophilic surface to easily remove dirt and stains [3 , 7]; 6) can decrease photochemical pollution by reducing ozone formation, particularly in the summer, which is the main cause of urban smog [3]; and 7) can mitigate the urban heat island effect, which helps to decrease the amount of energy required for cooling buildings and improve the air quality [3, 8].
The use of cement and TiO2 contributes well to NOx reduction [9, 10, 11]. The TiO2 loading technique and substrate characteristics play important roles in the TiO2 photocatalytic activity when mixed with cementitious materials [9]. Studies reported that favourable NOx degradation was obtained when the photocatalytic concrete substrate was rough [12].
There are different methods to prepare concrete with titanium dioxide [13]. A cement paste coating mixed with 5% TiO2 can efficiently remove NOx with more susceptibility to abrasion than other methods [14].
TiO2 has three main crystal forms: Anatase, rutile, and brookite. Anatase shows the most photocatalytic activity because the band gap energy is 3.2 eV, and the conduction band location is more favourable for driving conjugate reactions that involve electrons [15, 16]. The pollutant decomposition mechanism on the TiO2 surface is shown in Fig. (1).
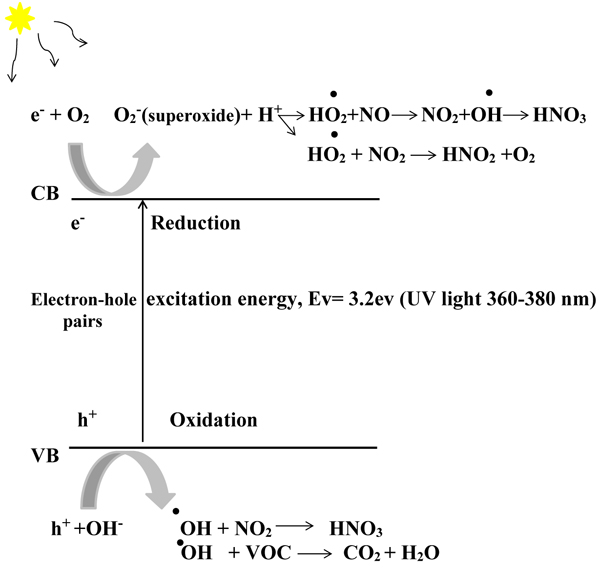
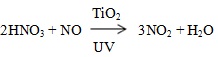
The accumulation of HNO3 on the photocatalyst surface promotes the conversion of NO to NO2 and reduces the NOx removal capability of the photocatalyst material [17]:
 |
(1) |
The photocatalyst can be renewed after rain (or washing) effects. A base such as TiO2-containing cementitious materials, whose pH values can be up to 13, enabled the formation of NO2– and NO3–. Hydroxyl radicals are produced in atmospheric chemistry via the reaction of excited atomic oxygen with water. The formation of OH and other oxygen-based species (e.g., peroxides, superoxides), is the crier for hydroxyl radicals and considered to be responsible for the mineralisation and oxidation of organics and inorganics. The TiO2 surface/colloidal chemistry and structure of TiO2 clusters in cement have significant effects on the photocatalytic performances. Because of the refinement effects of nT, the numerous but smaller pores increase the available surface area for adsorption and reaction of notably small molecules such as nitrogen oxides, which are better degraded by nT. Large molecules such as RhB dye, which hardly penetrate such a matrix, can easily penetrate into larger pores associated with micro-size TiO2: mT [18].
The hydration reaction is determined by the active sites on the cement-TiO2 particles. When nT particles are added to the cementitious matrix, they become potential nucleation sites for cement hydration products [19].
Many studies investigated the effect of moisture on photocatalytic NO degradation. Factors such as the photocatalytic materials, experimental conditions and initial NO concentration are influential in relation to the moisture humidity conditions. However, their roles remain notably controversial [20]. Some researchers reported that NOx conversion increased with increasing relative humidity [21, 22], whereas others concluded that the photocatalytic activity linearly decreased when the relative humidity increased [12, 23, 24]. Some studies illustrated that variation of the relative humidity between 40 and 70% did not induce any significant variation in the NO degradation rate [25]. No significant effect of the humidity was observed when the initial NO concentrations were 400 and 1000 ppb. When the NO concentrations increased to 2000 ppb and humidity decreased, a significant decrease in the degradation rate was observed [20].
The binding series for NO and NO2 with different w/c ratios have been investigated, and a conclusion is made that a higher w/c ratio affected the amount of gas that can be sorbed and oxidised. In addition, the amounts of gases absorbed by the pore solution are negligible compared to the gas bound on the hardened cement paste [26]. The hydroxyl groups for NO oxidation are originated from the water vapour in the air. In the case of zero relative humidity RH, the water content inside the catalyst and oxygen molecules in the air are the determining parameters for oxidation. A higher water content improved the catalyst photocatalytic activity [27].
The PC matrix is notably susceptible to the water-cement ratio (w/c) because of the effects on its pore structure properties. A lower w/c decreases the matrix porosity in terms of size and continuity, which reduces the gas transport properties. The photochemical degradation of some chemical compounds was seriously delayed because of the lack of water vapour in the matrix pores [15]. The addition of nanoparticles to cement paste leads to reduce the formation of hydration products which may lead to increase the connectivity of pore structure [28].
Another application of the cementitious photocatalytic TiO2 material is related to the removal of volatile organic compounds (VOCs). Some reports noted that the photocatalytic degradation of VOCs depended on both air humidity and initial pollutant concentration [20]. Others studied the removal of toluene from air [29]; they reported that high relative humidity and high inlet concentration led to low toluene removal performance, whereas better performance occurred at increased residence time.
In determining the self-cleaning performance for the photocatalysis to degrade stains and dirt under different illuminations, it was stated that UV and strong halogen light irradiation showed faster discolouration than weak illuminations [19]. Increasing the titanium dioxide level from 2% to 5% did not change the discolouration behaviour, which suggests that this process is controlled by the photon absorption instead of surface chemistry kinetics [19].
Micro-TiO2 performed better than or at least identical to nano-TiO2 because of the large particle agglomerate pores; the small and highly dispersed agglomerates of micro-TiO2 offer a higher available surface area for adsorption and reaction [18].
Porosity and pore pH/humidity in concrete pores could cause hydrolysis and improve the discolouration ability of the cementitious substrate, particularly in the presence of nT as a photocatalyst [30].
This study focuses on evaluating the effect of humidity on the photocatalytic NO degradation of PC composites that incorporate TiO2 nano- and micro-particles introduced in the bulk of the final cementitious layer. The natural atmospheric effects on the NOx and VOC removal ability of the PC samples were evaluated. Toluene was selected to represent the VOC species because of its abundance in the urban atmosphere [19]. Furthermore, the self-cleaning ability of the TiO2-modified composites was examined by applying a drop of methylene blue dye to the specimen surface and noticing the differences in dye discolouration with time under different illuminations. In terms of industrial applications, the findings of this study can provide insight into new nano-sized TiO2 photocatalytic material applications in cement-based finishing layers as an alternative to its predecessor, which is micro-sized TiO2.
2. EXPERIMENTAL PROCEDURES
The experimental programme is designed to assess the Photocatalytic Efficiency (PE) by comparing the performance of cement-based materials with various percentages and sizes of commercially available photocatalytic TiO2 particles.
2.1. Materials and Samples Preparation
Ordinary Portland Cement (OPC) type I, which has a Bogue composition of 58.80% C3S, 19.14% C2S, 7.95% C3A, and 9.42% C4AF and complies with ASTM C150-15, was used to prepare the samples.
Two grain sizes of commercial titanium dioxide powder were used: the micro-size (150-200 nm) from Tianjin Zhi Yuan Reagent Co., Ltd.-Chinese market with a specific surface area of 6.9 m2/g and the nano-size PC105 (20±5 nm) with a specific surface area of 85.6 m2/g from Cristal Active Millennium SAS- France.
Three sets of cement paste were prepared: one for the commercial nano-TiO2, one for the commercial micro-TiO2 and one as a reference sample. Two levels of TiO2 replacements were used for each commercial TiO2 product of 3% and 6%; a constant w/c ratio of 0.5 was used for all samples, according to preliminary results and another reference [26].
Each TiO2 powder was initially dispersed in deionised water and mixed for 2 minutes using a magnetic stirrer and another 2 minutes using a hand-held mixer. Then, cement was added, and the mixing was conducted according to ASTM C305 [31], except that the duration of mixing time was increased to 3 minutes to ensure thorough mixing and dispersion of TiO2 in the cement. To replicate conditions in field applications as closely as possible, no special procedures or chemicals were used to disperse the nano- or micro-TiO2 particles. The pastes were cast in 9-cm-diameter petri dishes to form 6-mm-thick samples, let harden for 24 h at room temperature, and cured for 28 days at 100% relative humidity. After 28 days of curing, the samples were conditioned in a 70° C oven until the weight stabilised and then stored under sealed conditions away from any light source until further testing.
The pollutant gases were from Mesa specialty gases and equipment, California. The concentrations were 400 ppm for both NO and toluene gases, which were diluted using another air cylinder (80% N2 and 20% O2).
2.2. Photocatalytic Removal and Measuring Procedure
The used laboratory reactor and schematic diagram of the experimental setup are represented in Figs. (2 and 3), respectively. The NOx photodegradation chamber and all other accessory components were designed according to the ISO 22197-1:2007 standard [32]. The NO inlet concentration and gas flow were modified to satisfy the research purposes and test requirements. The photodegradation chamber had a rectangular shape with dimensions of 30 cm x10 cm and was made of non-absorbing plastic. The system was completely sealed to prevent inadvertent leakage of the NO gas/air mixture. The gas detector device was from RAE Systems by Honeywell-USA, San Jose. The detection limits were 0-250 ppm for nitric oxide, 0-20 ppm for NO2 and 1-1000 ppm for VOCs. This device is simpler than the ordinary gas analyser used by others [18, 25] and is intended to facilitate the experimental setup with lower costs but sufficient accuracy as stated elsewhere [33].
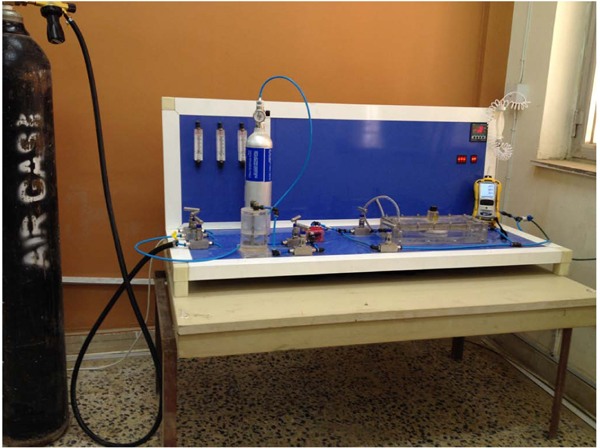
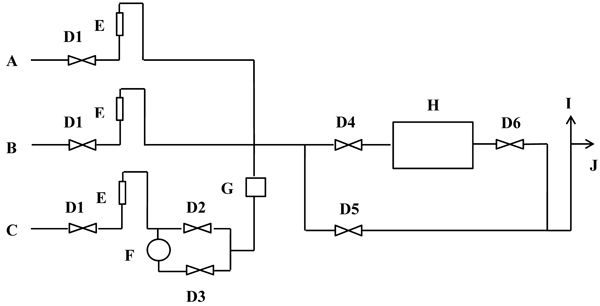
All tests were performed at room temperature with applying a continuous air flow at approximately 1.6±0.2 l/min. Two 6-W UV lamps were used with an intensity of 19 W/m2 per lamp and a wavelength range of 300-400 nm. The gas detector must be calibrated before each test. A piece of PC sample was placed inside the chamber, which was then tightly closed. Continuous airflow was ejected inside the reactor for approximately 1 h to clean the reactor, particularly the VOCs, with the UV lamps turned off to maintain dark conditions. The inlet for gas pollutant was opened and calibrated till the desired concentration (1 ppm for the NO test); for the toluene removal test, two concentrations were selected (3 ppm and 5 ppm). For both tests (NO and toluene removal) the humidity of the reactor was calibrated through control valves by using a humidifier to obtain the desired RH. In all test conditions, a waiting period of 25 min was maintained before the UV source was turned on to ensure chamber saturation and reading stabilisation. Upon starting, the reaction continued for approximately 62 ±2 min. Then, UV was turned off, the pollutant gas valve was closed, and only air flow was ejected into the chamber. A minimum of three replicated specimens were tested for each condition for all mix designs considered.
The calculation details of the NOx removal have been described elsewhere [17, 34]. The NOx removal is calculated by subtracting the amount of generated NO2 from the amount of removed NO. The calculations are as follows:
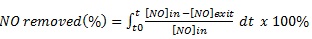 |
(2) |
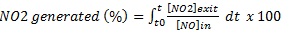 |
(3) |
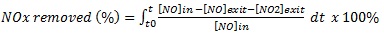 |
(4) |
where [NO]in and [NO]exit are the measured levels of NO gas stream before and during the UV irradiation, and t is the time in minutes.
2.3. Self-cleaning Performance
A surface observation to assess the photocatalytic degradation capability was performed by depositing a drop of methylene blue dye solution on the surface of the PC samples. The disappearance of the surface colour and variation of the stained area under illumination were monitored to determine the photocatalytic capacity. Photographs were taken before and after 10 h of exposure to recognise the potential development of stains with time. Three types of illuminations were applied to the TiO2 -modified PC specimens, and the three groups were compared. The first group was exposed to sunlight as a natural outdoor energy source that simulates the atmospheric outdoor conditions; the second group was exposed to a halogen lamp as a strong-energy visible light of 2000 W (21000 Lux); and the last group was exposed to 3 UV lamps, each of which had 6 W and an intensity of 19 W/m2 to provide a wavelength of 388 nm, which was sufficient to activate TiO2 [16]. The degradation of the deposited dye was compared after 10 h of illumination to investigate the degradation ability in different types of illumination conditions.
2.4. The Effect of Weather Humidity
Most cementitious finishing layers are frequently exposed to various atmospheric variables that mainly depend on the geographical zone or the season. In Middle East countries, such as Iraq, Jordan, Iran and Saudi Arabia, summer temperatures can reach 50o C, where even the shaded areas are warm and dry; these conditions continue for several months during the year. Consequently, photocatalytic cementitious materials may show different performances because of the notably low air humidities or high temperatures during the life cycle in such hot regions.
To assess the atmosphere effects on the photocatalytic efficiency of TiO2-modified PC composites, the prepared specimens were conditioned as follows before the photocatalytic efficiency (PE) test.
- Normal Conditions (NC): place the test specimens for four days in an ambient temperature of 23±2 °C and 50% relative humidity (RH) so that all faces are sufficiently aerated. For this purpose, the specimens were tested in March.
- Saturated Conditions (SC): immerse the test specimens in water for 48 h at 20±2 °C. For this purpose, the specimens were tested in February. The specimens were tested upon removal from water and in a saturated surface dry condition (SSD).
- Oven-Drying Conditions (DC): dry out the test specimen in an oven at 102°C until the difference between two consecutive weighings does not exceed 0.1% of the mass at 2 h intervals. Then, test the specimens in an ambient temperature of 40±2 °C. For this purpose, the specimens were tested in July.
3. RESULTS AND DISCUSSION
3.1. Photocatalytic Removal Capacity of TiO2-modified PC Samples
The NO and toluene removal efficiencies of the TiO2-modified PC samples are studied under UV irradiation and subjected to different humidity conditions (50%,100% and 0%). Reference samples showed no removal for the pollutant gases so the removal was noticed to be zero in all conditions.
3.1.1. Normal Conditions
In this case, the samples were subjected to a relative humidity (RH) of 50%; the temperature and flow rate were almost constant. NO/NO2 profiles are shown in Fig. (4) and the reaction performance for NO and NOx removal and NO2 generation is illustrated in Fig. (5). Although the removal efficiencies for both 3% and 6% mT appear equal, the NO2 concentrations generated from the 3% mT during the reaction are is greater than the amounts generated from the 6% mT sample with less removal for the NOx amounts. The PC specimens with nano-replacements appear to behave better than the micro-replacement, with good NOx removal. The fast reduction in Fig. (4c) in 3% nano-TiO2 samples can be explained by the fast adsorption of gas molecules by the hardened cement paste in addition to the photochemical reaction, even though the adsorption is of small amounts [26].
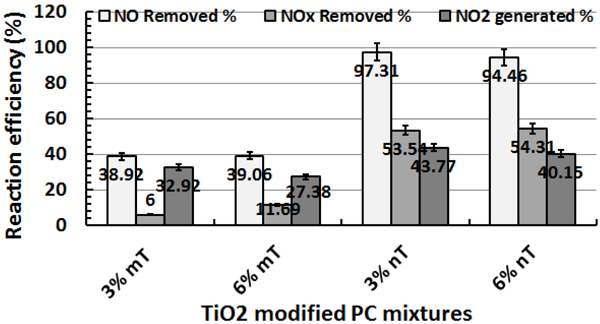
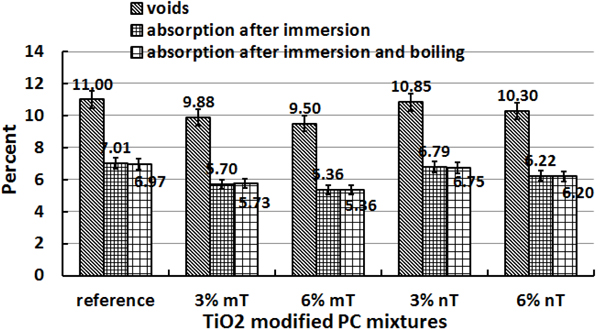
The reaction efficiencies indicate that the removal capacity improvements expected with 6% nT replacement were not clearly evident compared to the 3% nT replacement. The PC specimens with the 6% nT replacement had 3% less NO removal and 1.5% more NOx removal than the PC specimens with 3% nT. This result contradicts the hypothesis that higher TiO2 concentrations will lead to better photocatalytic reaction effects, as reported by another researcher [35]. To explain the results, the water absorption on the control and TiO2-modified PC specimens was tested on three additional disks of each PC mixture according to ASTM C642 [36] to measure the variations in porosity because of the mT or nT replacement. The results are presented in Fig. (6), the absorption test reveals that the PC specimens modified with TiO2 had lower absorption values than the control specimens. The nT replacement in this study actually increased the void percentages and water absorption of the mixtures compared to their counterparts of mT mixture. Higher replacement levels further reduced the voids and absorption results. The observed photocatalytic reaction behaviour may be related to this increase in the voids and absorption by using nT. This behaviour can be explained by the high concentration of TiO2 particles bound with cement paste, which formed a dense solid mass layer [19], increasing the percentages of TiO2 may reduce the reaction activity due to particles agglomerations. The reaction is on the surface instead of being in the inner part of the particles.
In the samples with 3% and 6% micro-replacements, the NO readings returned to 1 ppm because of the saturation with NO gas and reduced PC mixture efficiency in the removal with time (Fig. 4). NO2 values significantly increased at the end of the reaction for the 6% micro-replacement (Figs. 4a and 4b), which confirms that in this stage, the material was saturated and worked as a converter instead of a remover [17]; the nano-replacements continued until UV was turned off without saturation, as shown in Figs. (4c and d) Table 1.
Regarding the toluene removal, no removal was noted for the low gas concentration (3 ppm) in the photocatalytic toluene degradation experiment. Increasing the toluene gas concentration to 5 ppm resulted in a removal of 12.5%, 16.9% and 17.6% for the PC specimens that contained 6% mT, 3% nT, and 6% nT, respectively. Other researchers were also noted similar behaviour result and they stated that increasing inlet pollutant concentration lead to increase the reaction rate [15]. Chen also reported that the reaction rates increased with increasing pollutants concentration until it reaches a certain limit the photocatalytic and then started losing its activity [37]. Fig. (7a) shows that more time was required for the removal by the 6% mT specimens and approximately the same time was required for the toluene removal by the 3% nT and 6% nT specimens (Figs. 7b and 7c), respectively). The toluene removal results for the TiO2-modified PC specimens in the normal condition test are summarised in Table 2.

3.1.2. Saturated Conditions
When the PC specimens are completely wet, the removal of NO concentrations was much higher for the 3% mT and 6% mT samples than for their nano-counterparts, with increasing amounts of NO2 generation due to the reaction in Fig. (8). In addition, the removal for the 3% nT and 6% nT samples was low with less generation of NO2 concentrations (Fig. 9). The decrease in removal capacity for the nano-TiO2-modified PC samples in the saturated conditions can be explained by the ultrafine TiO2 particles being bound with water. A high TiO2 replacement level (i.e., 6%) with a large particle size (i.e., micro-size) improves the reaction in wet conditions compared to similar replacement levels with nano-size TiO2. The PC specimens with 3 and 6% mT replacements had 7.5, and 31.5% more NO removal, respectively, than the identical replacements levels of nT as shown in Fig. (9).
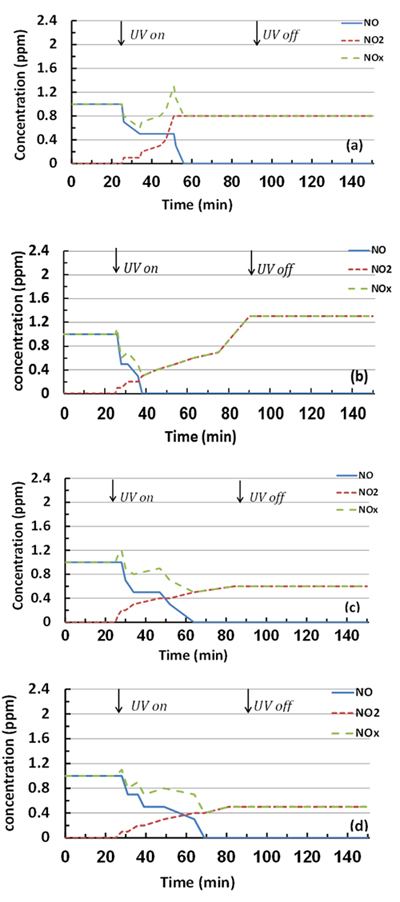
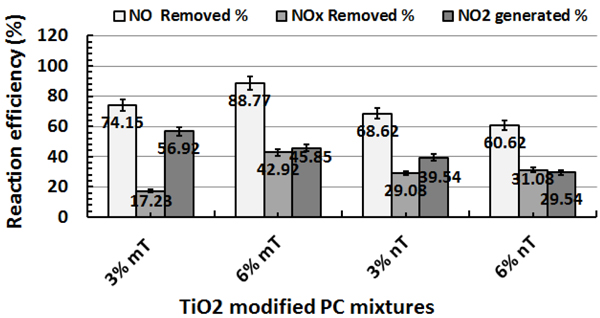
Fig. (8) shows that saturated samples require more time to recover to their inlet NO concentrations when UV is turned off. Thus, the reaction is with water-bound molecules instead of adsorption with the cement-based material, and this reaction requires more time to recover than the dry samples [26].
There was no removal by the saturated samples of toluene gas at low or high inlet concentrations (Table 2), which may be because of the low adsorption capacity of the physically or chemically bound water in the hydrated cementitious material [19]. Generally, the presence of excessive water competes with pollutants, which reduces the photochemical reaction for the PC mixture.
3.1.3. Dry Conditions
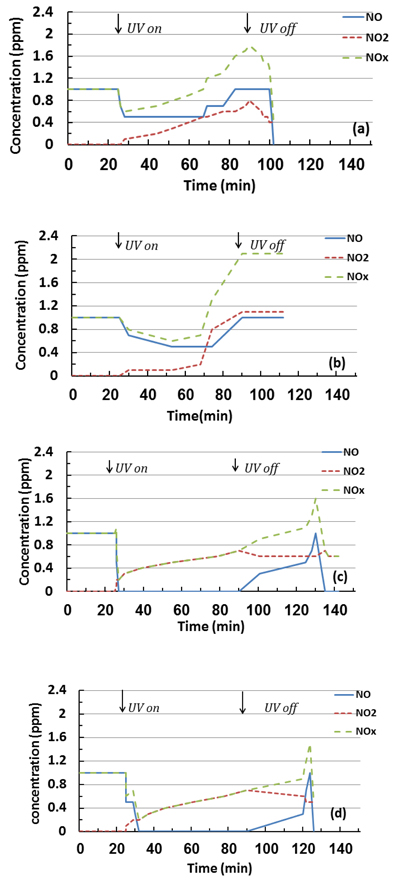
The effect of dry conditions on the photocatalytic reactions and removal efficiencies based on NO and NOx removal and NO2 generation during the test period is shown in Figs. (10 and 11). As observed, a sharp reduction in NO concentrations appeared when UV was turned on for all studied specimens. This fast reduction shows that the behaviour of TiO2 in the removal is enhanced without water molecule competition, and the oxidation occurred because of the reaction with oxygen molecules in the air [27], by generating the superoxide radicals. Furthermore, the PC specimens with 6% mT were more efficient in the removal than the 3% mT one, and the PC mixture was saturated with pollutant gas before the reaction ended for both 3% mT and 6% mT replacements, as shown in Figs. (10a and b), respectively.
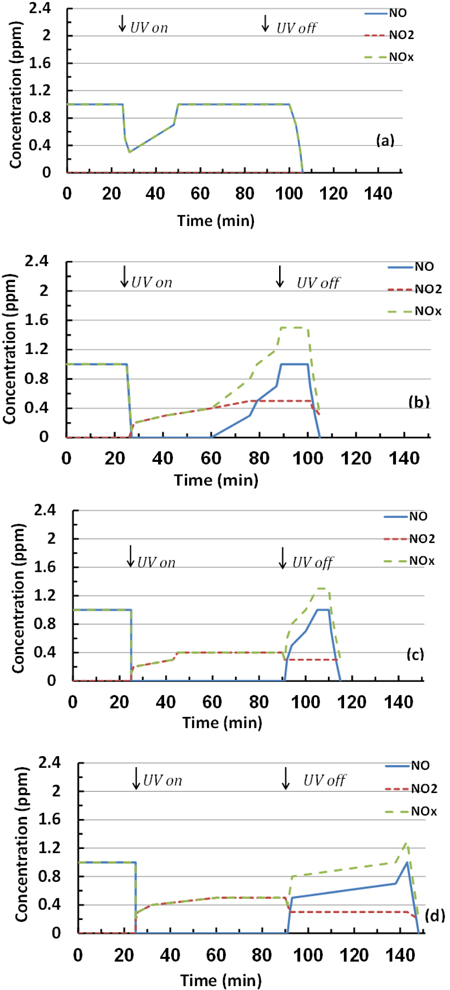
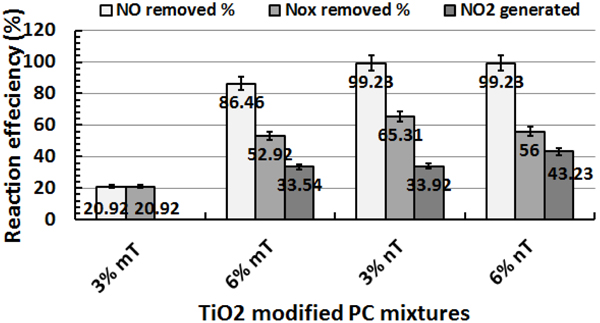
The use of nT significantly improved the NO removal efficiency, as represented by an increase of specimen bar removal percentages in Fig. (11). The PC specimens with 3 and 6% nT replacement had 375 and 14.5% more NO removal, respectively, than the same replacements levels of mT. In the case of NOx removal, the identical replacements had 212.2 and 5.8% more removal than the mT specimens, respectively. The removal efficiency of the PC specimens with nT replacement shows that the nT-containing specimens exhibited notably similar levels of removal efficiency, which indicates that higher levels of nT replacement are not essentially proportionally valuable.
More NO2 was generated by the 6% nT specimens, as shown in Fig. (11), which indicates that a higher concentration of nT replacements leads to more reaction under dry conditions.
The toluene test results in Table 2 and Fig. (12) shows a lack of removal efficiency for all TiO2-modified PC mixtures except the mixtures modified with 6% nT and tested at 5 ppm gas concentration. The removal was 13.79%, as shown in Table 2. Fig. (12 ) confirms the reduced reaction with water molecules of the gas, which easily returned to its inlet concentration when UV was turned off.
| Material | NO Removed (%) | NO2 Generated (%) | NOx Removed (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | SC | DC | NC | SC | DC | NC | SC | DC | |
| 3% mT | 38.92 | 74.15 | 20.92 | 32.92 | 56.92 | 0 | 6 | 17.23 | 20.92 |
| 6% mT | 39.06 | 88.77 | 86.46 | 27.38 | 45.85 | 33.54 | 11.69 | 42.92 | 52.92 |
| 3% nT | 97.31 | 68.62 | 99.23 | 43.77 | 39.54 | 33.92 | 53.54 | 29.08 | 65.31 |
| 6% nT | 94.46 | 60.62 | 99.23 | 40.15 | 29.54 | 43.23 | 54.31 | 31.08 | 56 |
| Material | Toluene Removed for 5 ppm Inlet Concentration (%) | Toluene Removed for 3 ppm Inlet Concentration (%) | ||||
|---|---|---|---|---|---|---|
| NC | SC | DC | NC | SC | DC | |
| 3% mT | 0 | 0 | 0 | 0 | 0 | 0 |
| 6% mT | 12.5 | 0 | 0 | 0 | 0 | 0 |
| 3% nT | 16.9 | 0 | 0 | 0 | 0 | 0 |
| 6% nT | 17.6 | 0 | 13.79 | 0 | 0 | 0 |
3.2. Self-cleaning performance
A methylene blue test was implemented in this study to observe the potential photodegradation and degree of lightening of the blue colour, which can be related to the photocatalytic oxidative behaviour of the PC specimens. Furthermore, the hydrophobicity of the PC mixtures can be evaluated by comparing the spot of the TiO2-modified PC specimens with the control. A wider area indicates higher hydrophobicity for the same deposited methylene blue solution volume. Figs. (13, 14 and 15) show the amount of dye degradation after 10 h of exposure to sunlight, halogen and UV illuminations, respectively. Even the PC specimens without TiO2 (i.e., the reference) had a degradation in colour, which highlights the importance of using a reference specimen to avoid misinterpretation [18]. In addition, the surface pigmentation area increased when the dye was exposed on the surface of the nT-modified PC specimens. This increase was more visible with the PC specimens that contained 6% nT. This behaviour can be interpreted as the surface hydrophilicity, which makes dirt and solid particles easily slip off. It is expected that a portion of the methylene blue dye penetrated into the PC matrix in proportion to the porosity of the matrix, which reduces the dye's expansion area. The observed performance of the PC specimens modified with nano-TiO2, which have more voids, as evident in Fig. (6), contradicts this expectation and confirms the hydrophilicity of this matrix.
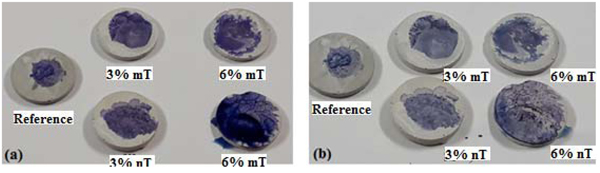
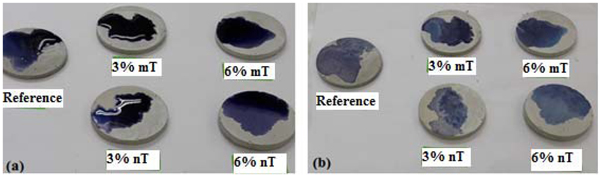
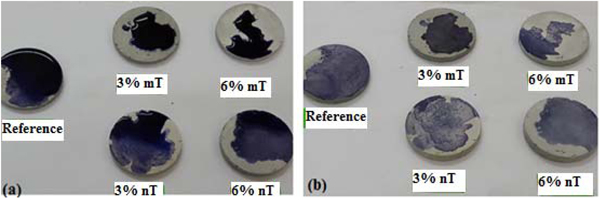
Furthermore, the discolouration for the dye was well noticed for the nT-modified PC specimens, which indicates better degradation for organics and stains that can be obtained using these percentage of replacements. These results contradict the conclusion of other research [18], where micro-TiO2 performs better or at least as well as nano-TiO2. The dispersion of nanoparticles with the cement matrix is the possible reason behind this phenomenon.
Although identical discolouration occurred for the used percentage by applying different types of illumination, the degree of discolouration differs from one type to another. The UV light shows faster discolouration than other illuminations [19]. Sunlight and halogen illuminations can cause bleaching because of the dye sensitisation and colour degradation.
CONCLUSION
This experimental comparison study of the photocatalytic efficiency and humidity effects on Portland cement mixtures containing different replacement levels of nanotitanium and microtitanium shows the following:
- The use of nT improves the photocatalytic efficiency of PC mixtures subjected to different moisture conditions. The levels of improvement for all PC mixtures with 3% or 6% nT replacements are relatively similar, which indicates that even small concentrations of nT replacement are almost as beneficial as larger concentrations of replacement.
- Weather humidity conditions strongly affect the PC mixtures modified with TiO2. In hot and dry conditions, better photocatalytic efficiencies are obtained by PC mixtures modified with nanotitanium. However, in saturated conditions, better efficiencies are obtained by PC mixtures modified with micro titanium.
- The matrix voids and surface absorptions significantly affect the activity of the PC mixtures, and increasing permeability promotes the ingress of the pollutant gas with the consequent enhancement in photocatalytic efficiencies. This performance confirms that the photocatalytic reaction is on the surface instead of being in the inner part of the particles, as mentioned elsewhere.
- An increase in the moisture content (from oven-dried to saturated conditions) of the PC mixtures modified with 3% or 6% mT improved the photocatalytic efficiency. However, this improvement was more noticed in the mixtures with lower mT concentrations 3% mT, the removal increased from 20.92 in the dry condition to 74.15 in the saturated condition. The removal performance was improved for the micro titanium mixtures because the large particle size can resist the amounts of excess water that are bound to the particles.
- The reduction in moisture content (from saturation to drying conditions) of nT-modified PC mixtures improved the photocatalytic efficiency. The negative effect of saturated conditions on the nano-titanium mixtures is because of excessive water amounts that compete with the gas and inhibit the reaction.
- Toluene was better removed in the moderate moisture condition, which is the normal state, because of the sharing of water molecules in the reaction, whereas in the saturation state, no removal appeared because the titanium particles bound with water molecules and prevented the reaction with toluene.
- In general, the replacement level of TiO2 particles effects appeared less important than the particle size impact (nano versus micro). PC mixtures with nano-titanium particles performed better than micro-titanium mixtures regardless of the concentration.
- The different illumination conditions in this study, UV light showed faster discolouration of methylene blue than other illuminations, as stated in other studies. Sunlight and halogen illuminations can cause bleaching because of the dye sensitisation and degradation of colour
LIST OF NOTATIONS
| eV | = Electron Volt |
| DC | = Dry Condition |
| NC | = Normal Condition |
| NO | = Nitrogen Oxide |
| NO2 | = Nitrogen Dioxide |
| NOx | = Nitrogen Oxides |
| mT | = Micro Titanium |
| nT | = Nano Titanium |
| nS | = Nano Silica |
| PC | = Portland Cement |
| PC105 | = Titanium Dioxide Produced by Cristal Active Millennium with a range size equals (20±5 nm) |
| RH | = Relative Humidity |
| RhB | = Rhodamine B Dye |
| SC | = Saturated Condition |
| UV | = Ultraviolet |
| VOC | = Volatile Organic Compound |
| w/c | = Water to Cement Ratio |
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors acknowledge the Building and Construction Engineering Department of University of Technology, Baghdad, Iraq, for extending the use of the facilities and laboratories for the above research work which is a part of the PhD thesis requirements. The authors would also like to thank Cristal Millennium for providing PC105 ultrafine titanium dioxide.


