RESEARCH ARTICLE
Utilization of Circulating Fluidized Bed Fly Ash as Pozzolanic Material
Kae- Long Lin1, *, Ta-Wui Cheng2, Chih-Hsuan Ho3, Yu-Min Chang4, Kang-Wei Lo2
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 176
Last Page: 186
Publisher ID: TOCIEJ-11-176
DOI: 10.2174/1874149501711010176
Article History:
Received Date: 26/9/2016Revision Received Date: 17/11/2016
Acceptance Date: 19/12/2016
Electronic publication date: 24/02/2017
Collection year: 2017

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A circulating fluidized bed (CFB) boiler generates energy by burning petroleum coke. Because burnt petroleum coke has a high sulfur content, limestone is added to the boiler to reduce the emittance of sulfur dioxide through desulfuration. The residue collected from the boiler is called CFB ash. CFB boilers in Taiwan can produce 328,000 tonnes of CFB fly ash per year. In this study, the pozzolanic characteristics of CFB fly ash were investigated by blending CFB fly ash and ordinary Portland cement (OPC). The CFB fly ash was mainly composed of CaO, SO3, and SiO2 in concentrations of 37.8%, 9.2%, and 2.2%, respectively. The crystals of CFB fly ash contained 3CaO.SiO2, 2CaO.SiO2, Ca(OH)2, C-S-H (Tobermolite), and Ettringite. The results revealed that applying the toxicity characteristic leaching procedure to CFB fly ash renders it suitable for use in blended cement. At later curing ages (90 days), the pore volumes of both the OPC and the CFB-fly-ash-blended cement pastes (CFBFABCP) decreased as the curing time increased. A possible explanation is that C3S and C2S were consumed to form C-S-H gel, resulting in an increase in the Q1 and Q2 groups identified by 29Si Nuclear Magnetic Resonance (NMR) spectroscopy. Furthermore, the peak of the Q0 group decreased, but those of the Q1 and Q2 peaks increased with an increasing curing time. The pozzolanic activity of the CFBFABCP containing 10% CFB fly ash indicates that it is a suitable substitute for OPC in blended cement.
INTRODUCTION
Circulating fluidized bed (CFB) combustion is a clean technology for coal burning, which has the advantages of adaptability to numerous fuels, high combustion efficiency, low NOx emissions, and stable operation over a wide range of load regulations [1]. CFB fly ash usually contains a high content of calcium, as both an oxide and a sulfate [2]. Numerous power stations use limestone as a sorbent for the SO2 released during coal combustion [3]. CFB fly ash differs from typical coal combustion by-products in that (1) it lacks the high content of f-CaO present in atmospheric fluidized bed combustion ash, thus preventing it from reacting vigorously with water, and lacks the high content of SO3 (usually anhydrite) present in both atmospheric fluidized bed combustion ash and pressurized fluidized bed combustion ash; (2) it consists of few spherical particles because the temperature in CFB combustion boilers (often 800-900 °C) is lower than that in pulverized coal-fired boilers (often 1300-1500 °C); and (3) it self-cements [4, 5]. CFB fly ash is potentially a valuable source of major oxides, such as CaO and SiO2, but technology for recovering CFB fly ash is unavailable. Approximately 800,000 tonnes of CFB fly ash has been dumped into landfills in Taiwan. Technological advancements would improve recovery and reduce the environmental impact of CFB fly ash.
Previous investigations have indicated that CFB fly ash along with pressured fluidized bed combustion ash or pulverized fuel ash can be used to prepare cementless concrete. Conn and Sellakumar [6] demonstrated that CFB ash can be used for construction applications that do not require highly stringent specifications, including soil stabilization, road base construction, structural filling, and synthetic aggregate production. Sheng et al. [1] investigation of the cementitious properties of fly ash produced by CFBC boilers cofiring coal and high-sulfur petroleum coke revealed that a high f-CaO content and a high SO3 content enhance cementitious properties; in other words, CFB fly ash is cementitious. Li et al. revealed that CFB fly ash has high pozzolanic reactivity and can be used as a cement admixture [7]. Chi et al. indicated that CFB fly ash can increase water absorption and effectively reduce initial surface absorption [3]. Additionally, CFB fly ash has a positive effect on the compressive strength, splitting tensile strength, and sulfate attack resistance of hardened, roller-compacted concrete. Previous investigations have indicated that the compressive strength of mortar comprising various combinations of coal-fired fly ash and CFB fly ash is lower than that of mortar containing Portland cement. However, coal-fired fly ash can effectively mitigate strength reduction [8].
The study focused on CFB-fly-ash-blended cement pastes (CFBFABCP), in which CFB fly ash is the cementitious material. The effects of CFB fly ash on the properties of CFBFABCP samples were investigated, and the relative influence of the CFB-fly-ash-to-cement ratio on the compressive strength and porosity of CFBFABCP samples were evaluated. Additionally, microstructural differences (degree of hydration and phase composition) among the CFBFABCP samples were examined.
MATERIALS AND METHODS
Materials
The CFB fly ash was obtained from a power plant in the Central Area of Taiwan. The CFB fly ash was homogenized; oven dried at 105oC for 24 hours; and then the chemical composition was characterized. The principal chemical compositions of the CFB fly ash were 37.8% CaO, 9.2% SO3, 2.3% Al2O3 and 2.18% SiO2. The leaching concentrations of the CFB fly ash met regulatory thresholds. Therefore, they have the potential as resources used for recycling. American Society for Testing and Materials (ASTM) Type I Portland cement from the Taiwan Cement Company was used in this study. It had a specific gravity was 3.15 and its physical-chemical properties met the requirements of ASTM C150.
Table 1 presents the total metals and leaching concentrations of raw materials. The total Ni metal level of the CFB fly ash was 345 mg/kg, and the second highest metal level was 36 mg/kg of Pb. The leaching concentrations of the raw materials met regulatory thresholds. Therefore, they have the potential as resources used for recycling.
| Curing Time (days) | OPC | 10% | 20% | 30% | 40% |
|---|---|---|---|---|---|
| 14 | 44 | 33 | 29 | 29 | 26 |
| 28 | 50 | 42 | 37 | 35 | 28 |
| 60 | 52 | 48 | 41 | 36 | 35 |
| 90 | 54 | 49 | 45 | 44 | 42 |
Tests Carried Out
Pastes using the aforementioned blends were prepared with a water to binder ratio of 0.4. 25.4 x 25.4 x 25.4 mm (1 x 1 x 1 in.) test cubes were prepared according to ASTM-305, followed by a moulding process (ASTM C31-69). The specimens were then demoulded and cured in a container at 95% humidity. After curing for 7, 14, 28, 60 or 90 days, the samples were subsequently crushed and the hydration reactions were stopped with absolute alcohol, filtrated in vacuum, washed with acetone and dried, then subjected by X-ray Diffraction, Mercury intrusion porosimetry (MIP), Differential Thermal and Thermogravimetric Analysis (DTA/TGA), Fourier-Transform Infrared (FTIR) spectrometry and 29Si magic-angle spinning/nuclear magnetic resonance (29Si MAS/NMR) analyses. Mercury intrusion porosimetry is based on the principle that the intrusion volume of mercury into a porous medium depends on the applied pressure. DTA/TGA analyses of the samples were performed using a Seiko SSC Model 5000 Thermal analyzer. Dry N2 gas was used as a stripping gas and a heating rate of 0.5°C was used. The samples were heated from 50 to 1000°C. FTIR spectrometry was performed using the KBr pellet technique (in which 1 mg of powdered sample was mixed with 150 mg KBr), by employing a Bomem DA8.3 FTIR instrument, and scanning from 2000 to 400 cm-1. The degree of hydration of Portland cement and CFBFABCP pastes were analyzed by using high resolution solid state 29Si MAS/NMR techniques as follows: The increase of diamagnetic shielding to the 29Si nuclei that resulted from the degree of increasing condensation from the single tetrahedral structure of the monosilicates (Qo) to the end groups (Q1), to the chain middle groups (Q2), to the layers and branching sites (Q3), and finally to the three-dimensional frameworks (Q4), led to well-separated and analytically useful chemical shift ranges for each type of SiO4 unit. The degree of hydration (designated as α) of the cement clinkers, C2S and C3S, can be evaluated as follows by the integral intensity of the signals at -70 ppm (Q0) for both the hydrated cement paste and cement powders, i.e., I0(Q0) and I(Q0), respectively [9, 10]:
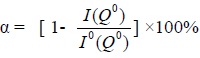 |
(1) |
RESULTS AND DISCUSSION
Compressive Strength Development of CFB Fly Ash Blended Cement Pastes
Table 1 shows the compressive strength development of the CFBFABCPs at the curing ages of 7, 14, 28, 60, and 90 days. The compressive strength of the ordinary Portland cement paste increased slightly with age, and the overall compressive strength was 44-54 MPa. As presented in Table 2, the compressive strengths of the CFBFABCPs were lower than that of the ordinary Portland cement paste on all curing days. The compressive strength of the ordinary Portland cement paste was inversely related to the CFB fly ash content of the mixture, although the compressive strength reduction was marginal when the CFB fly ash ratio in the mixture was high. At early curing ages, the compressive strength decreased with an increase in CFB fly ash because the pozzolanic activity of CFB fly ash was comparably lower. The compressive strength also decreased because the mixture contained less cement, thus less C3S. The reduction of C3S slowed hydration and reduced the rate of compressive strength development in the CFBFABCP samples. At the curing age of 90 days, the ordinary Portland cement paste had the highest compressive strength (54 MPa), followed (in descending order) by the CFBFABCP containing 10%, 20%, 30%, and 40% CFB fly ash (49, 45, 44, and 42 MPa, respectively). The results also revealed that when the CFBFABCP contained 10%-40% CFB fly ash, the corresponding compressive strength decreased by 5.6%-22.2%. Compressive strength development increased at later ages in the CFBFABCP samples than in the ordinary Portland cement pastes, particularly at higher concentrations of CFB fly ash. The decrease in compressive strength resulted from the reduction of the cement content of the mixture and excessive production of Ca(OH)2 from the reaction of free lime with water, which is unfavorable to the mechanical properties of cement [11, 12].
| Sample | Curing Time (days) | Weight Loss (%) | ||
|---|---|---|---|---|
| 105-440°C | 440-580°C | 580-1007°C | ||
| OPC | 7 | 23.22 | 8.39 | 11.83 |
| OPC | 28 | 24.28 | 8.73 | 14.98 |
| OPC | 90 | 28.68 | 12.71 | 8.42 |
| 10% | 7 | 23.11 | 8.37 | 9.11 |
| 10% | 28 | 25.66 | 9.14 | 12.43 |
| 10% | 90 | 27.72 | 10.92 | 12.10 |
| 20% | 7 | 19.90 | 7.35 | 13.65 |
| 20% | 28 | 25.92 | 10.24 | 9.26 |
| 20% | 90 | 26.44 | 9.47 | 12.59 |
| 30% | 7 | 23.28 | 10.09 | 8.18 |
| 30% | 28 | 24.94 | 9.24 | 13.13 |
| 30% | 90 | 26.12 | 9.27 | 16.17 |
| 40% | 7 | 23.70 | 8.99 | 11.71 |
| 40% | 28 | 23.83 | 6.69 | 17.76 |
| 40% | 90 | 24.01 | 8.37 | 18.18 |
Thermal analysis of CFB Fly Ash Blended Cement Pastes
TGA and differential thermogravimetry were used to monitor the hydration of the CFBFABCP samples during the 90-day of curing. For the CFBFABCP samples (Fig. 1), weight loss was observed at approximately 100-200 °C. The weight loss at approximately 400-580 °C can be attributed to Ca(OH)2 [13, 14], which was probably produced during the hydration reactions of the CFBFABCP samples. Other peaks observed for the CFBFABCP samples were located at approximately 580-1007 °C, to be attributed to the decomposition of CaCO3.
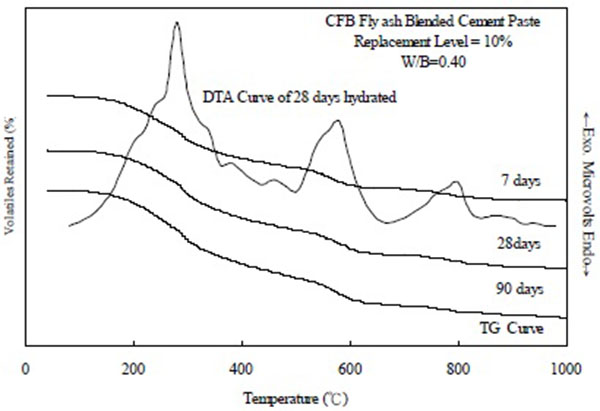 |
Fig. (1). TGA/DTG of CFB fly ash blended cement pastes. |
As presented in Table 2, a relatively high amount of C-S-H was detected in all CFBFABCP samples. The amount of C-S-H in the CFBFABCPs increased with age. At the curing age of 28 days, the C-S-H levels were higher in the CFBFABCP containing 10% and 20% CFB fly ash (25.66% and 25.92%, respectively) than in the ordinary Portland cement paste (8.73%). In the CFBFABCP containing 30% and 40% CFB fly ash, the C-S-H levels decreased as the CFB fly ash levels increased. This is usually associated with the C-S-H gel decomposition and its gradual increase indicated the increasing formation of this phase during hydration (Table 2). Conversely, the second peak at approximately100-200°C can be attributed to the dehydration of the CFBFABCPs. The difference in CH grains possibly resulted from the hydration of the different CFBFABCP samples. The peak position of the dehydroxylation temperature of Ca(OH)2 was reportedly influenced by crystallite size; smaller crystals were associated with lower peak positions [15, 16]. At the curing age of 90 days, the Ca(OH)2 concentration in the CFBFABCP containing 10%, 20%, 30%, and 40% CFB fly ash were 10.92%, 9.47%, 9.27%, and 8.37%, respectively, which were all lower than that of the OPC paste (12.71%). Therefore, the concentration of Ca(OH)2 continually decreased as the CFB fly ash concentration was increased to 40%.
FTIR Spectra of CFB Fly Ash Blended Cement Pastes
Fig. (2) illustrates the FTIR spectra of the CFBFABCP samples at various curing times. The band at a characteristic wavenumber of approximately 815-980 cm-1 decreased, indicating that alite (Ca3SiO5) dissolved [17, 18]. The peaks at 510-535, 864, and 936 cm-1 could be assigned to γ-C2S, whereas those at 883, 903, and 996 cm-1 could be attributed to the presence of β-C2S [19]. The CFBFABCP containing 30% and 40% CFB fly ash displayed weak Si-O bending vibrations at early ages because the content of the C3S mineral decreased, but α-C2S increased, as the amount of CFB fly ash increased. Thus, the hydration of α-C2S could not contribute to the Si-O bending vibrations. In addition, the poorly resolved band at around 500-750 cm-1 was attributed to C3A.
The band at a wavenumber characteristic of the -OH in Ca(OH)2 (approximately 3440-3640 cm-1), as well as the band at approximately 1424-1436 cm-1, was attributed to carbonate vibrations (C-O). The band at 1112 cm-1 was attributed to ν3 SO42- (sulfate) vibrations. By contrast, the band at 970-990 cm-1 was attributed to Si-O in the C-S-H gel. It subsequently shifted to 460, 525, and 900 cm-1, indicating Si-O (C3S) bending vibrations. Water stress (O-H) and deformation (H-OH) bands became visible at approximately 3500 and 1600 cm-1, respectively. Substantial changes were observed at approximately 1100-900 cm-1 between 28 and 90 days of curing. Presumably, the band observed at approximately 950-980 cm-1 was engendered by the formation of a C-S-H gel in activated pastes. Moreover, the Si-O bending vibrations of the C-S-H gel decreased as the CFB fly ash increased. When CFB fly ash was added to the pastes, the C3S mineral decreased, causing the C-S-H gel to decrease as well. The spectral bands of the CFBFABCP samples indicate the presence of water molecules from the crystallization or absorption of the reaction products. Moreover, changes also occurred at lower frequencies (in the range of the Si-O-Si deformation vibrations); the peak of 510-530 cm-1 shifted to 490 cm-1 and that of 580 cm-1 to about 560 cm-1 and were assigned to the deformation vibration of Si-O-Si [20-22].
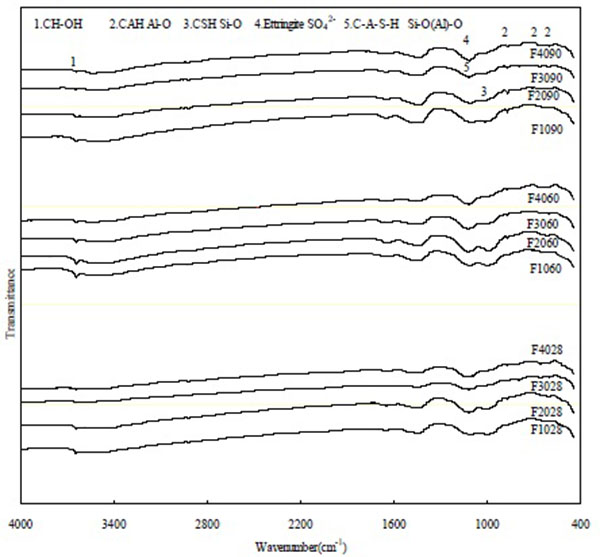 |
Fig. (2). FTIR spectra of the blended cement pastes at various curing times. |
Pore Size Distribution and Pore Volume of CFB Fly Ash Blended Cement Pastes
Mercury intrusion porosimetry (MIP) normally underestimates porosity, because the smallest pores, and some large pores, become separated from the interconnected pore network by the smallest pores and are thus inaccessible by mercury [23]. Nevertheless, the pore distribution was measured using the MIP method and the results are presented in Fig. (3). When pore size distribution decreases with an increase of curing time. When the pore size distribution of the CFBFABCP samples decreased from 10 to 1 μm, the pore size distribution curves shifted toward smaller pore sizes, indicating a finer pore structure; additionally, porosity slightly increased. Moreover, at early curing ages, the pore size distribution increased with the CFB fly ash replacement level; this increase was attributed to the comparably lower pozzolanic activity of CFB fly ash. At 90 days of curing, a further reduction in pore size distribution occurred, from 1 to 0.1 μm.
 |
Fig. (3). Pore size distribution of CFB fly ash blended cement pastes. |
Fig. (4) illustrates the pore volumes of the CFBFABCP samples at curing ages of 28 and 90 days. The observed pore volume decreased as the curing time increased. At the age of 28 days, the total pore volumes of the CFBFABCP samples containing 10%, 20%, 30%, and 40% CFB fly ash were 0.176, 0.191, 0.211, and 0.224 mL/g, respectively, which were all higher than that of the ordinary Portland cement paste (0.165 mL/g). The pore volume of the CFBFABCP samples exceeded that of the ordinary Portland cement paste and increased directly with the CFBC fly ash concentration at all ages. At the age of 90 days, the total pore volumes of the CFBFABCP samples containing 10%, 20%, 30%, and 40% CFB fly ash were all higher than that of the ordinary Portland cement paste (0.109 mL/g) at 0.136, 0.190, 0.198, and 0.215 mL/g, respectively. The total pore volumes continually increased as the CFB fly ash concentration was increased to 40%. This observation suggests that for the CFBFABCP containing 100% CFB fly ash, the hydration of the CFB fly ash particles would consume existing cement hydrates and produces new hydrates, maintaining a fairly constant porosity level while aging.
 |
Fig. (4). Pore volume of CFB fly ash blended cement pastes. |
29Si MAS/NMR Spectroscopy of CFB Fly Ash Blended Cement Pastes
Figs. (5 and 6) depict the 29Si MAS/NMR spectroscopy of ordinary Portland cement and the CFBFABCP containing 10% CFB fly ash. As shown by the 29Si NMR spectroscopy, the Q0 (-70 ppm) species shifted to the Q1 (-80 ppm) and Q2 (-87 ppm) species during curing [24]. As the curing time increased, the peak of the Q0° group decreased, whereas those of the Q1 and Q2 hydrates increased. Compared with the spectra of unreacted ordinary Portland cement, a major shift of the spectrum was detected. The 29Si NMR spectroscopy showed that the Q0 (-70 ppm) species shifted to the Q1 (-80 ppm) and Q2 (-87 ppm) species in the CFBFABCP containing 10% CFB fly ash, faster than that in the CFBFABCP containing 40% CFB fly ash. This is possibly because the CFBFABCP containing 10% CFB fly ash had a higher C3S mineral concentration than did the CFBFABCP containing 40% CFB fly ash, causing further production of C-S-H gel. According to these results, the conclusion was made that the mechanical strength of cement pastes obtained through alkaline activation of CFBFABCPs result from the formation of three different products, independent of curing conditions. The main reaction product is a low-crystalline calcium silicate hydrate with a dreierketten-type structure [25]. Similarly, the CFBFABCP containing 30%-40% CFB fly ash exhibited low transformation because the C3S mineral content decreased, but β-C2S and α-C2S increased as the amount of CFB fly ash increased. The hydration in early curing ages was mainly provided by C3S [26], whereas the α-C2S mineral did not provide any hydration. Thus, the conversion of the Q0 (-70 ppm) species into the Q1 (-80 ppm) and Q2 (-87 ppm) species was slowed considerably.
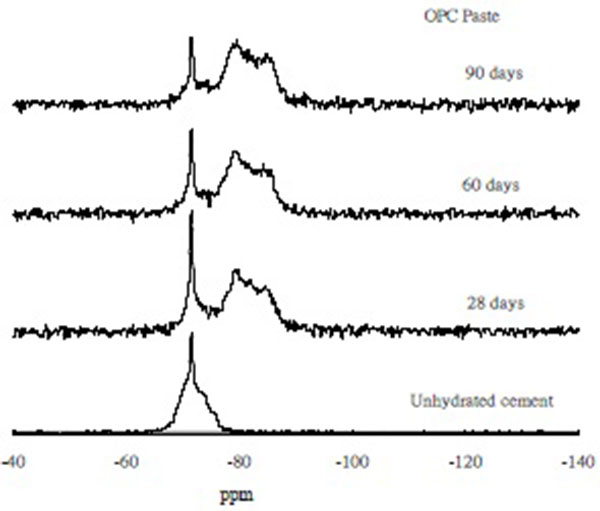 |
Fig. (5). 29Si MAS/NMR spectra of OPC. |
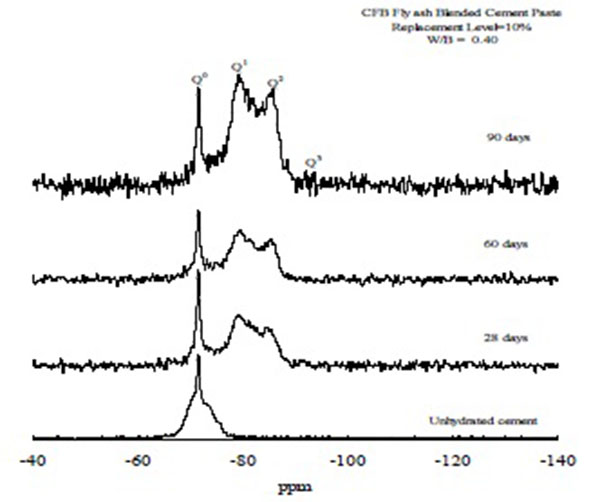 |
Fig. (6). 29Si MAS/NMR spectra of CFBFABCP containing 10% CFB fly ash. |
Table 3 shows the degree of hydration of the CFBFABCP samples. When the CFBFABCPs contained 10%, 20%, 30%, and 40% CFBC fly ash, the degree of hydration was 78.4%, 65.8%, and 52.3%, respectively. All the CFBFABCP samples displayed a lower degree of hydration than that of the ordinary Portland cement paste. It is obvious that the degree of hydration is able to reduce as the CFBC fly ash. The result was identical to that of the 29Si MAS/NMR spectroscopy. At 90 curing days, the degree of hydration of the pastes containing 10%, 20%, 30%, and 40% CFBC fly ash was 78.4%, 65.8%, and 52.3%, respectively. The results obtained by 29Si MAS/NMR confirmed that calcium silicate hydrate was a reaction product in the CFBFABCP samples.
| Sample | Curing Time (day) | 29Si NMR spectra | Total | Degree of Hydration (%) | |||
|---|---|---|---|---|---|---|---|
| Q0 | Q1 | Q2 | Q3 | ||||
| OPC | 28 | 363 | 326 | 253 | 59 | 1000 | 63.7 |
| OPC | 60 | 315 | 382 | 265 | 38 | 1000 | 68.5 |
| OPC | 90 | 295 | 374 | 287 | 44 | 1000 | 70.5 |
| 10% | 28 | 352 | 339 | 259 | 50 | 1000 | 64.8 |
| 10% | 60 | 326 | 376 | 274 | 24 | 1000 | 67.4 |
| 10% | 90 | 253 | 381 | 322 | 44 | 1000 | 74.7 |
| 20% | 28 | 408 | 313 | 235 | 44 | 1000 | 59.2 |
| 20% | 60 | 316 | 362 | 288 | 34 | 1000 | 68.4 |
| 20% | 90 | 307 | 349 | 305 | 40 | 1000 | 69.3 |
| 30% | 28 | 393 | 314 | 238 | 55 | 1000 | 60.7 |
| 30% | 60 | 310 | 351 | 298 | 41 | 1000 | 69.0 |
| 30% | 90 | 307 | 345 | 306 | 41 | 1000 | 69.3 |
| 40% | 28 | 396 | 315 | 234 | 55 | 1000 | 60.4 |
| 40% | 60 | 316 | 351 | 283 | 50 | 1000 | 68.4 |
| 40% | 90 | 307 | 325 | 308 | 60 | 1000 | 69.3 |
CONCLUSION
The main findings of this study are summarized as follows. CaO, SO3, and SiO2 were the major components observed in the CFB fly ash. For the CFBFABCP samples, the pore volume generally decreased as the curing time increased. An explanation for this observation is that at later ages (90 days), C3S and C2S were consumed and C-S-H gel was produced. Thus, the Q1 and Q2 hydrants increased, as identified by the 29Si NMR spectroscopy. Furthermore, the peak of the Q0 group decreased, but that of the hydrates Q1 and Q2 increased with the curing time. The degree of hydration of the CFBFABCP samples was lower than that of the ordinary Portland cement paste at the age of 90 days. Finally, the pozzolanic activity of the CFBFABCP with 10% CFB fly ash indicates that it is a suitable substitute for ordinary Portland cement in blended cement.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.







