Service Life Prediction of Inhibitors and Concrete Surface Coatings in Concrete-Embedded Reinforcing Steel Corrosion Caused by Chloride
Abstract
Background
Inorganic corrosion inhibitors and surface coatings have a number of drawbacks, such as high costs, toxicity to the environment, poor degradation, and environmental pollution. Green plant-derived corrosion inhibitors are being investigated extensively as a potentially useful substitute for conventional toxicity inhibitors.
Objective
In this study, we have tried to employ Calotropis gigantea and Azadirachta indica as environmentally friendly inhibitors to improve the chloride-induced corrosion resistance of reinforcing steel in concrete. The inhibitor efficiency of environmentally friendly inhibitors has been compared with chemical inhibitors and surface coatings.
Methods
The half-cell method and the linear polarization resistance method have been used to investigate the corrosion inhibition properties of steel bars embedded in cylindrical concrete specimens with different types of green inhibitors, chemical inhibitors, and surface coatings.
Results
The addition of Calotropis gigantea and Azadirachta indica leaf extracts to fresh concrete demonstrated a positive impact on corrosion resistance with control mixes. The efficiency of corrosion resistance increased with chemical inhibitors and surface coatings. Corrosion resistance has been found to be higher in concrete samples coated with surface coatings. From the results, surface coating has been found to exhibit the highest efficiency than the chemical and green inhibitors.
Conclusion
Overall, the study has demonstrated that, in cases of chloride contamination, surface coatings, particularly polyurethane ones, can provide protection against rebar corrosion. Organic inhibitors have shown promise as non-toxic, environmentally benign substitutes, despite their lower effectiveness.
1. INTRODUCTION
Nowadays, Reinforced Concrete (RC) is the most widely used building material. Its popularity can be attributed mainly to its favourable engineering qualities and low cost when compared to other building materials, like steel or wood. Millions of people throughout the world depend on these structures for their way of living, and their early or sudden failure can have catastrophic effects on the economy, waste time, and occasionally even result in fatalities [1, 2].
This is mostly regarded as an economic issue as opposed to merely an engineering one. The annual cost of repairing steel bars corroding in buildings and bridges in the United States is estimated to be $150 million [3].
Industries have created a variety of corrosion prevention techniques to prevent corrosion loss. Among them are corrosion inhibitors, which are widely employed in many industries due to their low cost, great flexibility, straightforward procedure, and economic effectiveness. The corrosion inhibitor can spare metal from ruining at incredibly tiny concentrations. The corrosion inhibitors can be used without altering the procedure or equipment and offer a number of benefits, such as rapid reaction, high efficiency, straightforward operation, low cost, and simplicity of use [4].
In acidic environments, the plant components' extracts have good inhibitory effects. They are also harmless, safe, and environmentally friendly. Conventional inhibitors are pricey, hazardous, and harmful to the environment. This is the reason why researchers are interested in corrosion inhibitors that are economical, environmentally benign, and extremely effective. Green corrosion inhibitors are becoming more and more of a popular idea. Drugs, roots, chitosan, oil, flour, yeast, natural honey, leaves, herbs, and so forth are examples of green corrosion inhibitors. Research claims certain organic and biodegradable substances to be quite powerful inhibitors. Plant extracts are rich in polar molecules having oxygen and nitrogen as well as non-polar compounds with aromatic rings, aliphatic chains, heterocyclic rings, and functional moieties. Unlike inorganic chemicals, these substances can be efficiently absorbed on the metal surface to prevent corrosion, thereby not endangering the environment [5, 6].
Furthermore, plants that include organic compounds, including alkaloids, flavonoids, heteroatoms, tannins, and nitrogen-based compounds, such as flowers, seeds, leaves, roots, and stems, might be a good source of corrosion inhibitors. Among these, heteroatom-containing organic compounds work well as corrosion inhibitors [7, 8].
Pradipta et al. [9] showed that, when applied to the corrosion of steel reinforcing bar (rebar) embedded in mortar, tea extract (GT) exhibited a greater Inhibition Efficiency (IE) at equal volume as commercial calcium nitrite corrosion inhibitor. GT created a protective coating on the rebar surface by acting as a mixed-type corrosion inhibitor and increasing rebar polarization resistance (Rp).
In the study by Dehghani et al. [10], the extract from Ziziphora leaves was used as a novel environmentally friendly green inhibitor to stop Mild Steel (MS) from corroding when exposed to acid. According to the Electrochemical Impedance Spectroscopy (EIS) analysis, the inhibitory performance increased to 93% at higher concentrations of Ziziphora leaf extract. The polarization curves showed that the extract from Ziziphora leaves delayed the corrosion of steel through mixed inhibition control.
In the work of Liu et al. [11], in order to lessen the corrosion caused by reinforcement, waste Platanus acerifolia Leaves (PAL) were used to create an environmentally friendly corrosion inhibitor. Electro- chemical tests have demonstrated the PAL extract to be a highly effective mixed-type inhibitor, inhibiting both cathodic and anodic processes.
Liu et al. [12], in their study, attempted to use ginger extract as an eco-friendly inhibitor to increase the corrosion resistance of reinforcing steel caused by chloride in solutions that simulated concrete pores. The ginger extract proved to be an effective corrosion inhibitor, according to electrochemical data, lowering chloride-induced corrosion at the ideal dosage (2%).
The outcomes of the experiment by Nabil Al-Akhras et al. [13] demonstrated Eucalyptus leaves to have the potential to be employed as environmentally friendly steel reinforcing corrosion inhibitors. Visual examination of the damaged RC beams revealed the amount of rust to be reduced as the EL concentration rose.
In the work of Zhang et al. [14], the mechanism by which Maize Gluten Meal Extract (MGME) inhibited steel corrosion was systematically determined by combining theoretical calculations with experimental research. The MGME and its primary basic constituents, Glu, Pro, and Leu, were found to be in charge of inhibition; the effectiveness of this inhibition has been found to be 83.15% and 79.27%, respectively.
In the study carried out by Alibakhshi et al. [15], in a 1M HCl solution, the Glycyrrhiza glabra extract has been utilized to inhibit mild steel. The extract from Glycyrrhiza glabra leaves acted as a mixed-type inhibitor.

2. MATERIALS AND METHODS
2.1. Materials
In this investigation, cylindrical specimens being 150 mm in height and 75 mm in diameter with steel reinforcement positioned in the center were created. A steel bar with a diameter of 12 mm was used to maintain an effective cover of 44 to 45 mm at the bottom of concrete examples that had reinforcing steel positioned at the center. Cement of OPC 53 grade was used. An effective water-to-cement ratio of 0.5 was used to create the concrete mixtures. The fine and coarse aggregates had specific gravities of 2.66 and 2.68, respectively. 20 mm maximum size of coarse aggregate was used in proportions of 60 and 40, respectively, in all concrete formulations. Water was absorbed by coarse aggregate at a rate of 2.8% and by fine aggregate at 0.57% [16].
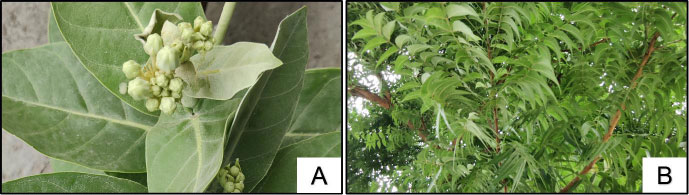
Two kinds of organic inhibitors were used: Azadirachta indica (AI) and Calotropis gigantea (CG) leaf extracts. The plants of Calotropis gigantea (CG) and Azadirachta indica (AI) are depicted in Fig. (1). Sodium molybdate dihydrate (Na2MoO4·2H2O) and benzotriazole, two inorganic inhibitors, were used to compare the effectiveness of organic inhibitors. Sodium molybdate dihydrate, an analytical reagent, and 99.0% pure benzotriazole were mixed in different amounts of 0, 1, 2, and 3% of cement. Two kinds of surface coatings were used: polyurethane and acrylic surface coatings. The schematic diagram illustrating the production of organic inhibitor is displayed in Fig. (2) [16].
2.2. Service Life Prediction
Utilizing corrosion protection devices and various concrete mixture designs in different proportions, Life-365 is a software that estimates the life-cycle costs and the service life of a mixture. The Life-365 Consortium I and II groups of companies developed a research-based methodology to provide estimates of the effects of surface barriers, steel types, chloride exposure, temperature, design, and high-performance concrete mixture propor- tions on the life-cycle cost and service life of steel-reinforced concrete structures.
When designing steel-reinforced concrete structures that will be exposed to chlorides, design consultants may find this straightforward and transparent model to be a crucial tool for assessing the life cycle costs and service life of alternative protective systems.
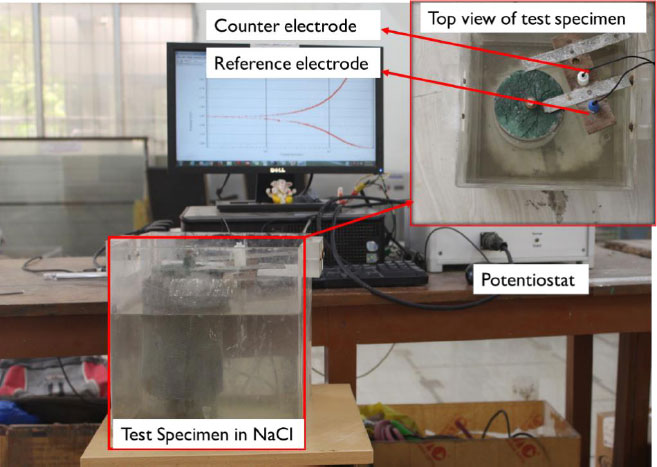
After casting, the specimens were covered with wet burlap for 24 hrs. After that, the specimens were demolded and cured for 27 days. The specimens were subjected to a chloride solution after being cured for 27 days. Until the test day, samples were partially immersed in 4% NaCl solutions (by volume of water). It provided the advantage of initially removing the dispersion of corrosion data caused by the concrete's dry conditions by permitting the moisture necessary for corrosion. The half-cell potential and linear polarization tests for corrosion have been carried out on reinforced concrete cylinder specimens on a regular basis. Potentiostat K-Lyte 1.2 was used to monitor the corrosion of reinforcing steel bar inserted in cylindrical concrete specimens both with and without four different types of inhibitors and surface coatings [1].
3. RESULTS AND DISCUSSION
3.1. Corrosion Measurements
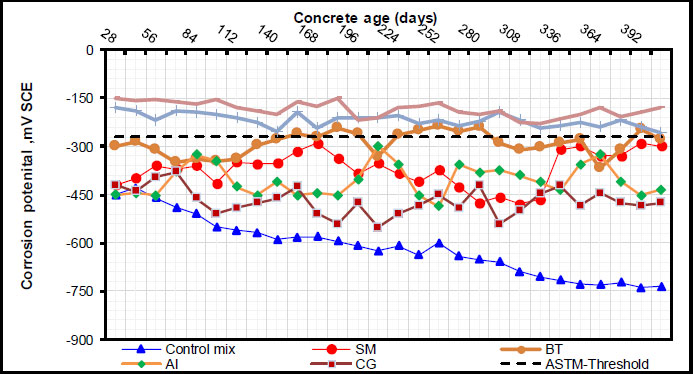
As can be seen in Fig. (3), the corrosion potential values were determined on cylindrical specimens utilizing rebar embedded in concrete as the working electrode and the Standard Calomel Electrode (SCE) as the reference electrode. As can be observed in Figs. (4 and 5), the RC specimen with inhibitors and surface coatings exhibited corrosion potential and corrosion current density values. In terms of inhibiting corrosion, inorganic inhibitors, like benzotriazole and sodium molybdate dehydrate, as well as green inhibitors, like CG and AI, demonstrated efficient outcomes. Results of current density and corrosion potential indicated that inorganic inhibitors outperformed organic inhibitors. In comparison to inorganic inhibitors, the organic inhibitors, CG and AI, demonstrated a greater degree of corrosion prevention, although their effectiveness was lower. Compared to samples mixed with CG, those mixed with AI demonstrated lower corrosion current density values and lower negative potential values at the same dose. This shows that AI was more successful at preventing the occurrence and the progression rate of rebar corrosion in concrete. It implies that, in contrast to specimens mixed with CG, those treated with AI persisted in a state of moderate corrosion activity for a longer amount of time. Overall, the study demonstrated that in cases of chloride contamination, corrosion inhibitors, particularly inorganic ones, can provide protection against rebar corrosion. Organic inhibitors have shown promise as non-toxic, environmentally benign substitutes, despite their lower effectiveness.
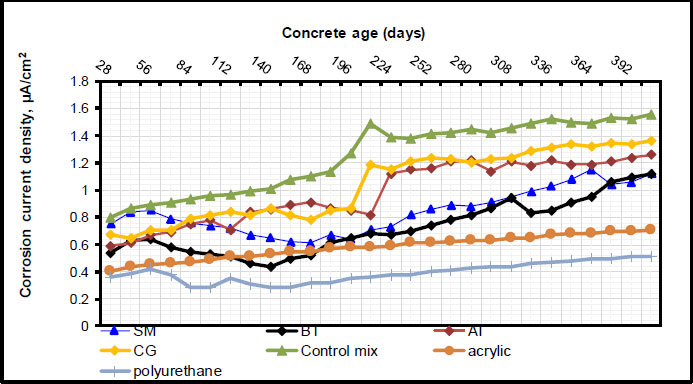
Promising outcomes were observed with surface coatings, specifically acrylic and polyurethane coatings. Results of current density and corrosion potential indi- cated that surface coatings outperformed all inhibitors. Comparing samples combined with all inhibitors, those with surface coatings showed lower values for corrosion current density and negative potential. This implies that surface coating is more successful at preventing the initiation and progression rate of rebar corrosion in concrete. The study has thus demonstrated that, in cases of chloride contamination, surface coatings, particularly polyurethane ones, can provide protection against rebar corrosion.
3.2. Service Life Prediction
3.2.1. Chloride Coefficient Test
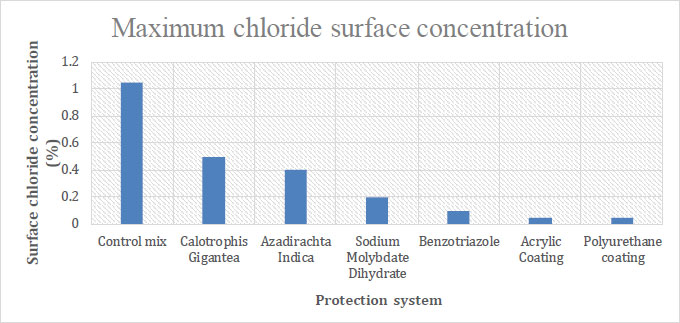
Concrete pore structure has a greater impact on chloride surface concentration. Fig. (6) shows the chloride surface concentration results for each specimen. It has been discovered that concrete with surface coatings exhibited a minimized chloride diffusion coefficient, leading to decreased porosity. Specimens lacking any form of protection had the highest chloride diffusion coefficient. Thus, the resistance of concrete with a surface coating to chloride ions was significantly increased, which in turn decreased the potential pace at which reinforcement could corrode.
Green inhibitors showed decreased porosity compared to the control mix, but they have been found to be less effective than the chemical inhibitors. Chemical inhibitors showed decreased porosity but were less effective than surface coatings.
3.2.2. Evaluation of Service Life of Concrete Using Life-365
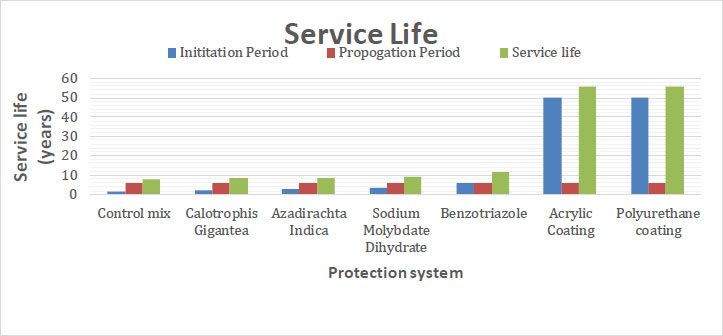
Fig. (7) shows the service life values of RC specimens with various protection setups. The impact of inhibitors and surface coatings on the service life of concrete was assessed using the Life-365 software. The specimens cast in the laboratory were used in this experiment. The surface chloride content, the chloride threshold, and the chloride diffusion coefficient from the Chloride Migration Coefficient Test (CMCT) were the input parameters used by Life-365 to determine the service life. The concrete's cover depth, which guards against chloride penetration, determines the extent of the first corrosion. As a result, the initial corrosion was determined using Life-365 software to match different concrete cover depths. Concrete without any kind of protection system had a service life of around 7.6 years, but concretes with surface coating, chemical inhibitors, and green inhibitors had longer service lives, about 56, 11.7, and 8.4 years, respectively.
| Sl No. | Protection System | Maximum Chloride Surface Concentration (%) |
|---|---|---|
| 1 | Control mix | 1.050% wt. conc. |
| 2 | Calotrophis gigantea | 0.500% wt. conc. |
| 3 | Azadirachta indica | 0.400% wt. conc. |
| 4 | Sodium molybdate dihydrate | 0.200% wt. conc. |
| 5 | Benzotriazole | 0.100% wt. conc. |
| 6 | Acrylic coating | 0.050% wt. conc. |
| 7 | Polyurethane coating | 0.050% wt. conc. |
| Sl No. | Protection System | Initiation Period (years) |
Propagation Period (Years) | Service Life (Years) |
|---|---|---|---|---|
| 1 | Control mix | 1.6 | 6 | 7.6 |
| 2 | Calotrophis gigantea | 2.1 | 6 | 8.1 |
| 3 | Azadirachta indica | 2.4 | 6 | 8.4 |
| 4 | Sodium molybdate dihydrate | 3.2 | 6 | 9.2 |
| 5 | Benzotriazole | 5.7 | 6 | 11.7 |
| 6 | Acrylic coating | 50 | 6 | 56 |
| 7 | Polyurethane coating | 50 | 6 | 56 |
According to the results provided by the Life-365 software, it can be concluded that the corrosion resistance of steel bars in reinforced concrete showed peak value with surface coating. Green inhibitors showed a positive impact on corrosion resistance, but were less effective than the inorganic inhibitors. The concrete's service life was extended when surface coating was incorporated. These findings suggest that adding a surface coating to concrete can greatly increase its resistance to salt damage (Tables 1 and 2).
CONCLUSION
Adding Calotropis gigantea and Azadirachta indica leaf extracts to fresh concrete exhibited a positive impact on corrosion resistance. The efficiency of corrosion resistance increased with the addition of chemical inhibitors and surface coatings. Corrosion resistance was higher in concrete samples coated with surface coatings.
Surface coatings, including acrylic and polyurethane coatings, showed promising results. The results showed that surface coatings performed better than green and chemical inhibitors in terms of both current density and corrosion potential.
The study findings have also shown that surface coatings, especially polyurethane ones, can prevent rebar corrosion in the conditions of chloride contamination. Despite their lesser efficiency, organic inhibitors have shown promise as being non-toxic and environmentally friendly.
Green corrosion inhibitors are now a distinct area of study in cement and concrete science. There is a great deal of variability when it comes to green corrosion inhibitors, and many of them have the potential to be useful and effective corrosion inhibitors. To evaluate their technical qualities, however, more research is required. This includes modeling functional groups for corrosion prevention and their efficacy against the combined attack of corrosive ions.
LIST OF ABBREVIATIONS
| SM | = Sodium Molybdate Dihydrate |
| BT | = Benzotriazole |
| CG | = Calotrophis Gigantea |
| AI | = Azadirachta Indica |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [Sreenivasa] at [https://zenodo.org/ records/10797290”], reference number [10797290]”.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


